The purpose of the second part of the experiment was to recrystallize our crude samples of benzoic acid and 2. Isolation of Caffeine from your first year chemistry labsYou will be separating a mixture of two compounds triphenylmethanol a neutral organic compound and.
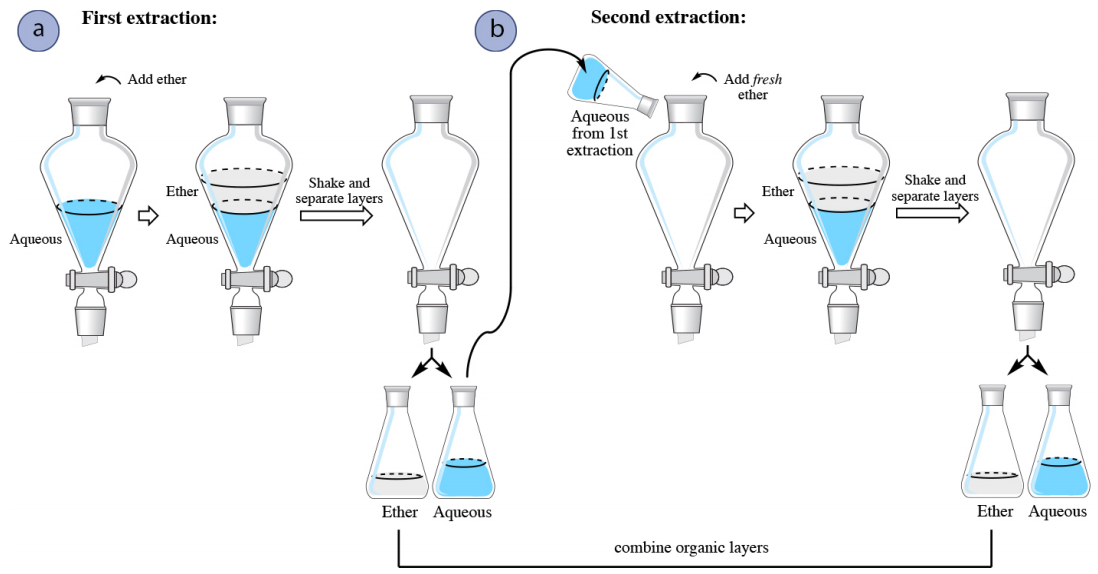
4 5 Extraction Theory Chemistry Libretexts
Cooling directly from gas to.

. Liquid-Liquid Extraction This experiment serves as a general introduction to the Chem. In a typical LLE experiment an aqueous sample is mixed with an apolar nonmiscible solvent like n-hexane. So we will move to second step in this step we will use deferents amount of solvent and solute to see how they effect on the process.
In this experiment you will use the common technique of liquid-liquid extraction to separate and purify benzoic acid and naphthalene from a mixture to the two. Going directly from a solid to a gas CO2 at -78 degrees C deposition. Department of Chemical Engineering Illinois Institute of Technology.
ABSTRACT SUMMARY In liquid-liquid extraction experiment it consists of two parts. Dissolving the mixture in the first solvent and then adding a. Liquid-liquid extraction we were able to recover 513 of benzoic acid and 520 of hydroquinone dimethyl ether and 83 of 2-naphthol using liquid-liquid extraction.
1 - 1 Experiment 1. Liquidliquid extraction LLE is based on the principle that a solute or an analyte can distribute itself in a certain ratio between two immiscible solvents usually water aqueous phase and organic solvent organic phase. How slow or fast they work and 4.
2-In all parts of the experiment the extraction happened we did not saw how the amount of solute and solvent has effect on the result. Liquid-Liquid Extraction This experiment serves as a general introduction to the Chem 235 Organic Chemistry Laboratories. The drying agent should not react with the solvent or any solutions present 2.
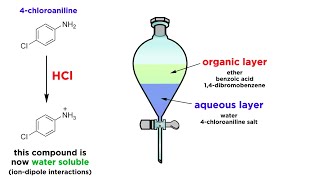
This technique can be used to separate covalent molecules from ionic compounds in an aqueous solution or suspension. Where ionic species are removed from a non-polar solvent by extraction into water. 1-The Liquid-Liquid extraction process it works fine.
1 - 1 Experiment 1. Experiment 4 - Liquid-Liquid extraction. Liquid-Liquid Extraction This experiment serves as a general introduction to the Chem 235 Organic Chemistry Laboratories.
The extraction technique can be used to purify compounds or to separate mixtures of compounds such as when isolating a product from a reaction mixture known as an extractive work-up. Sample Calculations 3-Discussion of Results 4-Appendices Appendix A Figures 5-. We will be building on the first year introductory extraction experiments ex.
A typical liquid-liquid extraction involves partitioning a mixture of compounds between two immiscible solvents. Know how much water will be left behind 3. It also has applications in the isolation of natural products as in the extraction of caffeine from tea leaves.
Where a solid or liquid suspended or dissolved in one solvent is extracted into another. In this experiment liquid-liquid extraction was successfully used to separate a mixture into three parts. Once the target analytes are in the organic phase they can be re-extracted into a fresh aqueous phase whose pH has been manipulated to ensure the analytes are in the charged form and therefore most highly.
We will be building on the first year introductory extraction experiments ex. Isolation of Caffeine from your first year chemistry labsYou will be separating a mixture of two compounds triphenylmethanol a neutral organic compound and. How much they cost.
Firstly to determine the distribution coefficient for the system organic solvent-Propionic acid-water and to show it dependence on concentration and the second is to demonstrate how a mass balance is performed on the extraction column and to measure the mass. The selectivity of the extraction experiment involving pH manipulation can be further improved using a technique known as Back Extraction. View Experiment 1- Liquid Liquid Extractionpdf from CHEM MISC at University of British Columbia.

Liquid Liquid Extraction Vs Solid Phase Extraction
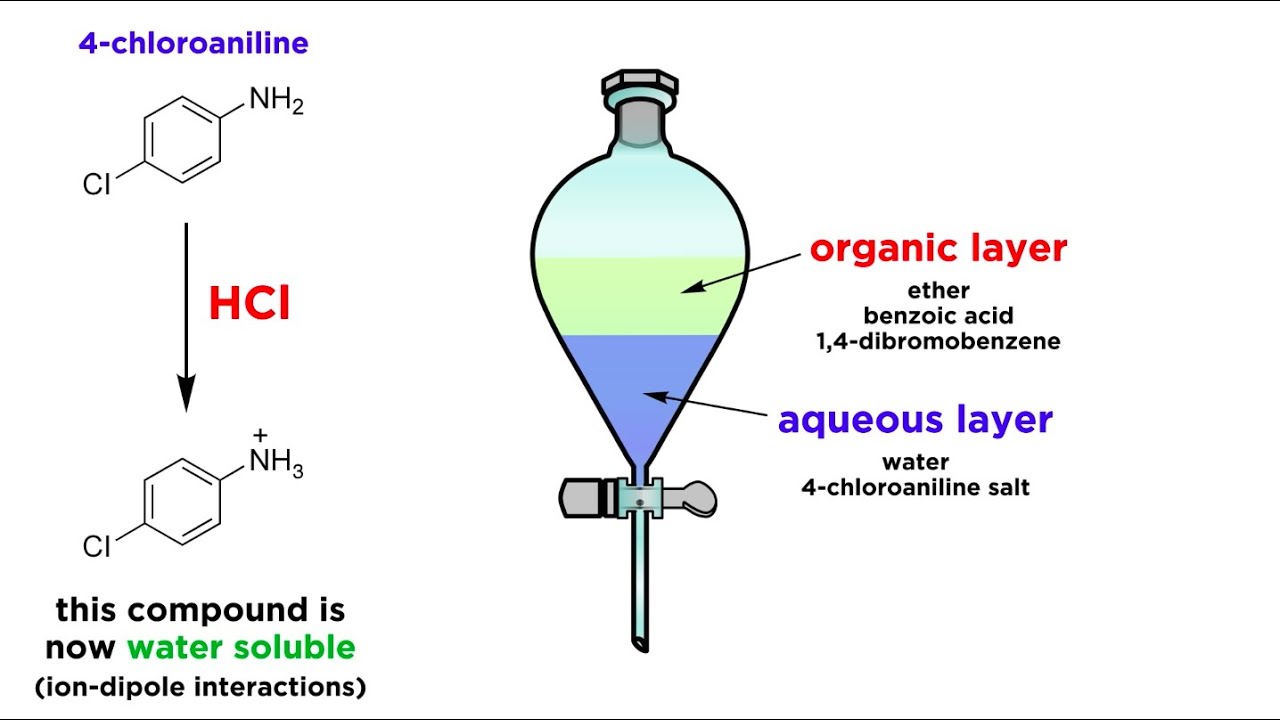
Separating Components Of A Mixture By Extraction Youtube
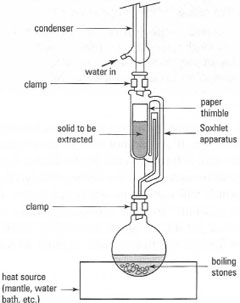
Solid Liquid Extraction Solvent Extraction Laboratory Techniques

Pressurized Solvent Extraction An Overview Sciencedirect Topics
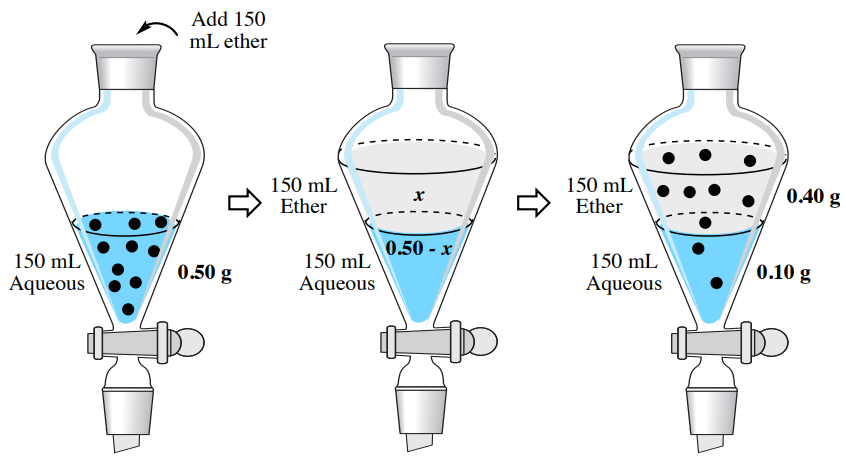
4 5 Extraction Theory Chemistry Libretexts
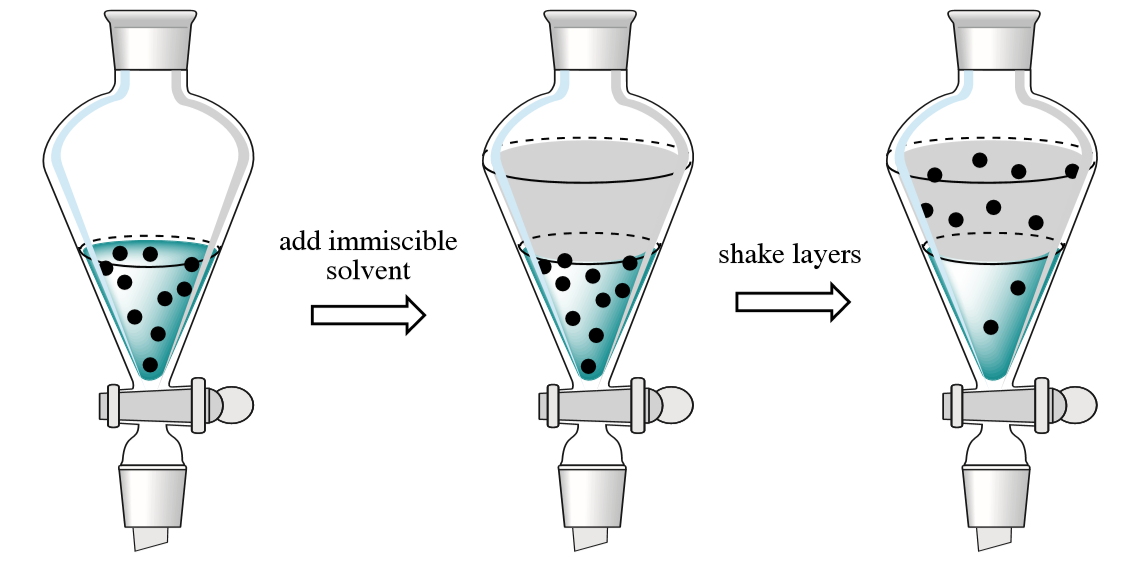
4 2 Overview Of Extraction Chemistry Libretexts

Liquid Liquid Extraction Vs Solid Phase Extraction
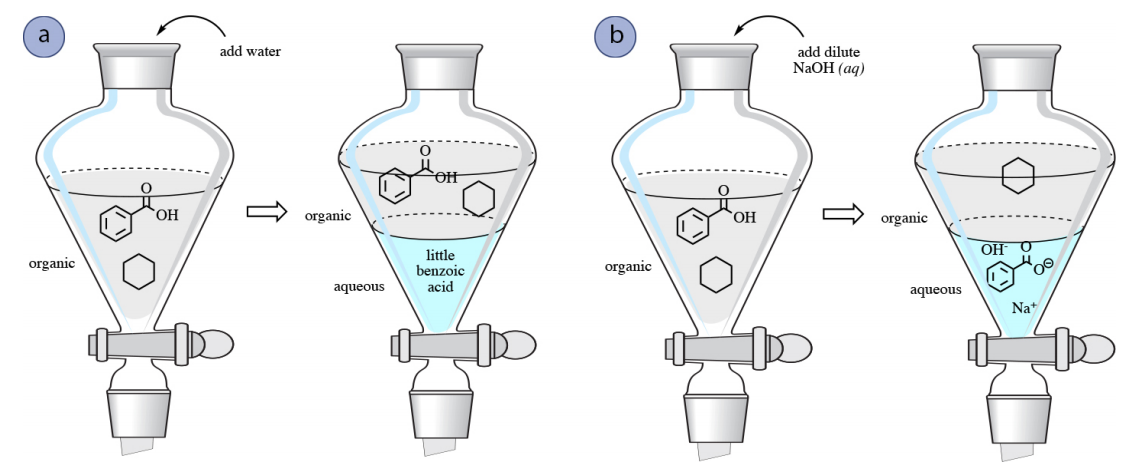
4 8 Acid Base Extraction Chemistry Libretexts
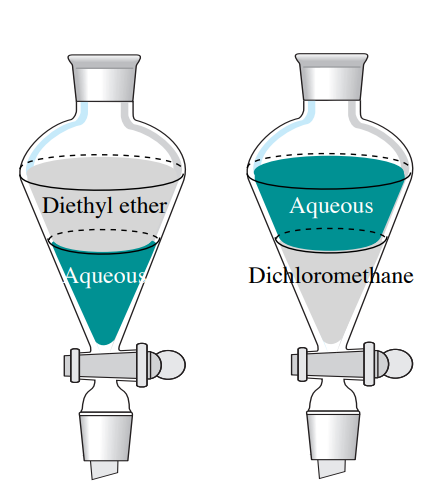
4 4 Which Layer Is Which Chemistry Libretexts
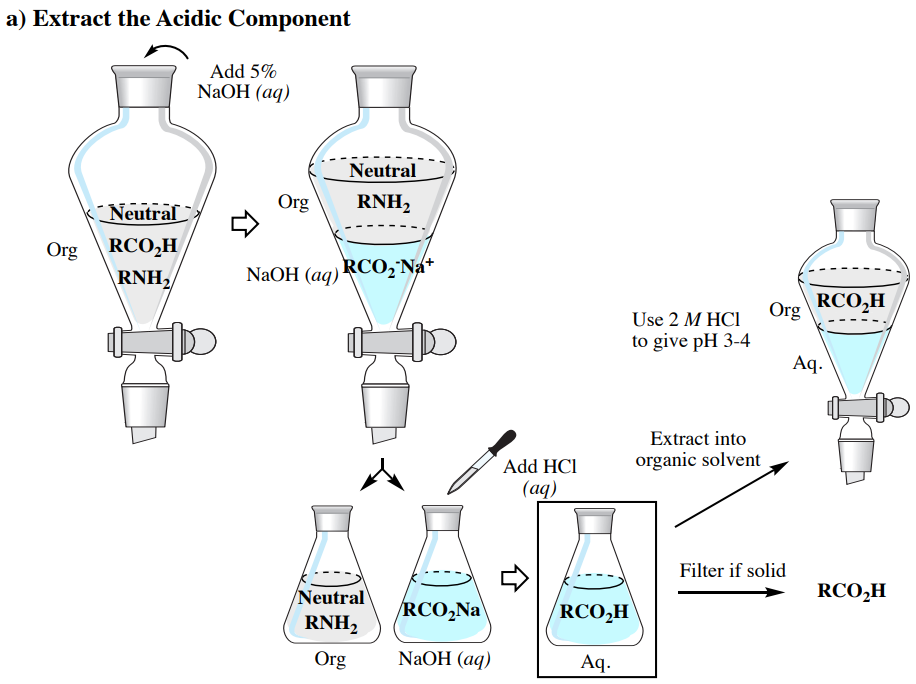
4 8 Acid Base Extraction Chemistry Libretexts

Liquid Liquid Extraction Vs Solid Phase Extraction
Separating Components Of A Mixture By Extraction Youtube

Solvent Extraction An Overview Sciencedirect Topics

Chem117 04 Liquid Liquid Extraction Fundamentals Youtube

Solvent Extraction Or Separation Youtube

Liquid Liquid Extraction Youtube
The Complete Guide To Hydrocarbon Extraction New In 2022

Liquid Liquid Vs Supported Liquid Vs Solid Phase Extraction
